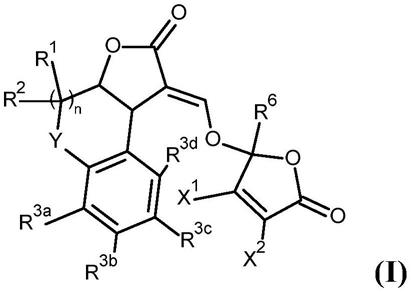
一类新型碳酸酐酶ix靶向光敏剂及其在医药领域的应用
技术领域
1.本发明属于化学医药技术领域,涉及光敏剂药物与光动力疗法,具体涉及一类新型碳酸酐酶ix(caix)靶向光敏剂及其在医药领域的应用。
背景技术:
2.光动力疗法(photodynamic therapy,pdt)是光激活以光敏剂为基础的治疗方法,已经被确定为针对多种肿瘤的消融安全模式(natrev clin oncol,2020,17,657-674)。有效光照开启能使治疗在空间和时间上高度可控且侵入性最小。目前临床pdt中,光激发的光敏剂通过与生物活性分子相互作用产生细胞毒性自由基(i型机制),或在ii型机制中,将基态氧(3o2)直接转化为高反应性的细胞毒性激发态单线态氧(1o2)。pdt不仅可以直接杀死肿瘤细胞,还会造成肿瘤新生血管损伤,阻碍肿瘤供氧。
3.光敏剂是pdt的关键成分。第一代光敏剂是一种低聚血卟啉衍生物(hpd)混合物,于1993年在加拿大被批准用红光(630nm)治疗膀胱癌,尽管其存在癌症选择性差、低红光吸收和长期光敏性等问题,但仍然是使用最广泛的光敏剂。5-氨基乙酰丙酸是原卟啉ix生物合成的关键前体,及其衍生物氨基乙酰丙酸甲酯易于高纯度合成,并被fda批准为第二代光敏剂。卟啉光敏剂的外周双骨架还原可以红移并增强其最大波长吸光度。因此,二氢卟酚衍生物foscan、radachlorin和laserphyrin已被批准用于红光(约 660nm)的临床pdt,而菌绿素衍生物redaporfin在临床ii期,可用红外光(749nm)激活。本团队许德余、张浩等早期研发了血卟啉单甲醚(中国医药工业杂志,1989,20(2):60-63),也称海姆泊芬(hmme,hemoporfin),其化合物成分明确、给药后避光期短等明显优点,成为全国首个治疗鲜红斑痣的光敏药物(中国激光医学杂志,2018,27(1):01-05),但血卟啉单甲醚仍存在组成成分为一对异构体、结构不是很稳定等缺点。
4.目前大多数pdt药物主要通过依赖氧的ii型机制起作用,这会导致肿瘤内严重缺氧。这反过来又会限制光动力疗法的治疗效果。pdt诱导的缺氧可能是由于光敏剂直接消耗氧气或通过脉管系统退化间接导致。pdt诱导缺氧的一个推论是它触发了缺氧诱导因子(hif)介导的信号级联反应。通过血管生成因子的转录激活,导致负责肿瘤进展的许多相关调节基因上调。编码碳酸酐酶ix(caix)基因属于hif通路上调的基因。caix调节细胞内和细胞外ph值,并促进缺氧环境中的肿瘤存活和侵袭。事实上,caix是侵袭性乳腺癌(例如mda-mb-231 肿瘤细胞)生存率低和远处转移的标志物。caix受肿瘤特异性过度表达的影响,在正常组织内表达高度受限,这一事实使其成为具有潜在吸引力的治疗靶点。最近,lou等人报道,抑制caix活性显著控制体内乳腺肿瘤的生长和转移(cancer res.,2011,71,3364-3376.)。在caix基因水平敲低和bevacizumab治疗后,在小鼠肿瘤模型中也观察到了增强的治疗效果(clin.cancerres.,2012,18,3100-3111)。kim等人报道乙酰唑胺偶联的bodipy光敏剂 (az-bps),通过结合抗血管生成治疗与pdt联合来减轻pdt导致的缺氧影响(j.am.chem. soc.2017,139,7595-7602)。
[0005][0006]
因此,本发明中我们使用噻二唑磺酰胺和苯磺酰胺(az)作为靶向碳酸酐酶ix(caix)基团与pdt相结合,即在本团队合成的光敏剂焦脱镁叶绿酸-a的羧基上引入caix受体小分子抑制剂噻二唑磺酰胺和苯磺酰胺,制备了系列焦脱镁叶绿酰胺衍生物:
[0007]
技术实现要素:
[0008]
pdt的功效与光敏剂的ros生成能力有关,其本质上依赖于氧气。肿瘤微环境中氧气的消耗会加剧肿瘤缺氧,并触发缺氧诱导因子(hif)介导的信号级联反应,进而激活一系列负责癌细胞的靶基因(如葡萄糖转运蛋白和血管内皮生长因子)生存、肿瘤发生和远端转移。为了解决这一问题,迫切需要消除pdt加剧缺氧的副作用,以提高其治疗效果。本发明设计合成了新的焦脱镁叶绿酰胺衍生物。研究表明噻二唑磺酰胺和苯磺酰胺系列化合物的引入,可以提高化合物的荧光量子产率和单线态氧产率,并增强化合物的光毒性效果。新化合物结构稳定,制作工艺简单易行。
[0009]
本发明涉及一类焦脱镁叶绿酰胺类衍生物及其应用。
[0010]
本发明概述如下:
[0011]
一类焦脱镁叶绿酰胺衍生物具体下述结构(式i)和(式ii)及(式iii):
[0012][0013]
其中,n为1,2,3,4,5,6,7,8;m为0-8的整数。
[0014]
本发明所述的焦脱镁叶绿酰胺衍生物(式i)和(式ii),该类化合物包括:
[0015]
n-(5-氨磺酰基-1,3,4-噻二唑-2-基)焦脱镁叶绿酰胺(i);
[0016]
n-[2-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-2-氧代乙基]焦脱镁叶绿酰胺(ii1);
[0017]
n-[3-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-3-氧代丙基]焦脱镁叶绿酰胺(ii2);
[0018]
n-[4-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-4-氧代丁基]焦脱镁叶绿酰胺(ii3);
[0019]
n-[5-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-5-氧代戊基]焦脱镁叶绿酰胺(ii4);
[0020]
n-[6-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-6-氧代己基]焦脱镁叶绿酰胺(ii5);
[0021]
n-[7-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-7-氧代庚基]焦脱镁叶绿酰胺(ii6);
[0022]
n-[8-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-8-氧代辛基]焦脱镁叶绿酰胺(ii7)。
[0023]
本发明所述的焦脱镁叶绿酰胺衍生物(式iii),该类化合物包括:
[0024]
n-((4-氨磺酰基)苯基)焦脱镁叶绿酰胺(iii1);
[0025]
n-((4-氨磺酰基)苯基甲基)焦脱镁叶绿酰胺(iii2);
[0026]
n-(2-((4-氨磺酰基)苯基)乙基)焦脱镁叶绿酰胺(iii3);
[0027]
n-(3-((4-氨磺酰基)苯基)丙基)焦脱镁叶绿酰胺(iii4);
[0028]
n-(4-((4-氨磺酰基)苯基)丁基)焦脱镁叶绿酰胺(iii5);
[0029]
n-(5-((4-氨磺酰基)苯基)戊基)焦脱镁叶绿酰胺(iii6);
[0030]
n-(6-((4-氨磺酰基)苯基)己基)焦脱镁叶绿酰胺(iii7);
[0031]
n-(7-((4-氨磺酰基)苯基)庚基)焦脱镁叶绿酰胺(iii8);
[0032]
n-(8-((4-氨磺酰基)苯基)辛基)焦脱镁叶绿酰胺(iii9)。
[0033]
本发明首次将碳酸酐酶ix小分子抑制剂类化合物引入焦脱镁叶绿酸a侧链,制备了上述新型焦脱镁叶绿酰胺衍生物(式i、式ii和式iii),该类光敏剂具备明显的靶向性、显著的光动力活性,可作为肿瘤、视网膜黄斑变性、光化性角化病、鲜红斑痣、尖锐湿疣等疾病的光动力治疗药物,具有实用性。
具体实施方式
[0034]
下面结合具体实施例,进一步阐述本发明。应理解,这些实施例仅用于说明本发明而不用于限制本发明的范围。此外应理解,在阅读了本发明讲授的内容之后,本领域技术人员可以对本发明作各种改动或修改,这些等价形式同样落于本技术所附权利要求书所限定的范围。
[0035]
[实施例1]
[0036]
n-(5-氨磺酰基-1,3,4-噻二唑-2-基)焦脱镁叶绿酰胺(i)的制备:
[0037][0038]
在圆底烧瓶中加入焦脱镁叶绿酸-a(107mg,200μmol),无水乙腈40ml,再加入1,3,4
‑ꢀ
噻二唑-2-磺酰胺(52mg,240μmol),然后加三乙胺(0.1ml,800μmol),室温搅拌30min。最后加入hatu(56mg,240μmol),室温搅拌反应16h,tlc监测反应完全后,加入100
ꢀ‑
200ml饱和氯化钠溶液,再加入2m hcl溶液调节ph至酸性。然后加入乙酸乙酯萃取(100 ml
×
3),水洗。收集有机相,用无水na2so4干燥,过滤,减压蒸除溶剂。对所得残留物进行柱层析分离纯化,得到绿色固体粉末状化合物i(74mg,53%)。1h nmr(600mhz, dmso-d6)δ:9.68(s,1h),9.40(s,1h),8.90(s,1h),8.20(dd,j=17.7,11.6hz,1h),7.24(s,2h), 6.38(d,j=17.8hz,1h),6.20(d,j=11.6hz,1h),5.24
–
5.08(m,2h),4.55(dd,j=7.4,2.2hz, 1h),4.33
–
4.25(m,1h),3.68(d,j=7.7hz,2h),3.61(s,3h),3.44(s,3h),3.19(s,3h),2.63
–ꢀ
2.57(m,1h),2.48
–
2.33(m,2h),2.21
–
2.13(m,1h),1.98
–
1.90(m,1h),1.74(d,j=7.4hz, 3h),1.61(t,j=7.6hz,3h),0.22(s,1h),-1.98(s,1h).
13
c nmr(151mhz,dmso-d6)δ:195.26, 172.24,171.07,161.98,153.55,149.47,147.81,144.06,140.52,136.80,135.31,134.89,134.51, 131.45,128.85,127.35,122.37,105.72,103.43,95.93,93.60,51.40,49.36,47.61,36.01,30.57, 22.93,18.32,17.29,11.89,11.49,10.49.hrms(maldi-tof)m/z:calcd for c
35h37
n8o4s2[m]
697.23737,found 697.23609.
[0039]
[实施例2]
[0040]
n-[2-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-2-氧代乙基]焦脱镁叶绿酰胺(ii1)的制备:
[0041][0042]
采用化合物i的合成方法制备了化合物ii1。1h nmr(400mhz,dmso-d6)δ:13.10(s,1h), 9.74(s,1h),9.46(s,1h),8.92(s,1h),8.38(s,1h),8.30(s,2h),8.29
–
8.17(m,1h),6.40(d,j= 17.8hz,1h),6.22(d,j=11.6hz,1h),5.25(d,j=20.2hz,1h),5.13(d,j=20.1hz,1h),4.61(d, j=7.6hz,1h),4.35(s,1h),4.06(s,2h),3.80
–
3.66(m,2h),3.63(s,3h),3.45(s,3h),3.23(s, 3h),2.67(s,1h),2.33(s,1h),2.20(s,2h),1.80(d,j=7.3hz,3h),1.64(d,j=7.5hz,3h),0.28 (s,1h),-1.94(s,1h).
13
c nmr(151mhz,dmso-d6)δ:195.28,172.76,172.13,170.01,164.03, 161.43,153.69,149.61,147.81,144.24,140.57,136.88,135.49,134.96,134.66,131.50,128.91, 127.50,122.52,105.87,103.63,96.11,93.61,51.23,49.38,47.55,42.50,32.29,30.21,22.90,18.38, 17.33,11.93,11.52,10.59.hrms(maldi-tof)m/z:calcd for c
37h40
n9o5s2[m]
754.25883, found 754.25598.
[0043]
[实施例3]
[0044]
n-[3-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-3-氧代丙基]焦脱镁叶绿酰胺(ii2)的制备:
[0045][0046]
采用化合物i的合成方法制备了化合物ii2。1h nmr(600mhz,dmso-d6)δ:13.00(s,1h), 9.65(s,1h),9.38(s,1h),8.88(s,1h),8.27(s,2h),8.18(dd,j=17.8,11.6hz,1h),8.07(s,1h), 6.36(d,j=17.9hz,1h),6.19(d,j=11.6hz,1h),5.21(s,1h),5.13(s,1h),4.53(d,j=7.7hz, 1h),4.25(d,j=8.9hz,1h),3.64(d,j=8.1hz,2h),3.59(s,3h),3.42(s,3h),3.41(s,1h),3.17 (s,3h),2.68(d,j=7.2hz,2h),2.61(q,j=5.5,4.9hz,1h),2.38(s,2h),2.12-2.08(m,2h),1.74 (d,j=7.4hz,3h),1.59(s,3h),0.18(s,1h),-2.03(s,1h).
13
c nmr(151mhz,dmso-d6)δ: 195.30,172.07,170.73,164.29,161.46,161.12,
147.82,144.34,140.57,136.95,135.59,134.71, 131.52,128.92,122.57,105.89,103.76,96.20,93.63,51.19,49.40,47.52,35.26,34.54,32.32, 30.21,22.83,18.40,17.34,11.92,11.52,10.61.hrms(maldi-tof)m/z:calcd for c
38h42
n9o5s
2 [m]
768.27448,found 768.27272.
[0047]
[实施例4]
[0048]
n-[4-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-4-氧代丁基]焦脱镁叶绿酰胺(ii3)的制备:
[0049][0050]
采用化合物i的合成方法制备了化合物ii3。1h nmr(600mhz,dmso-d6)δ:12.94(s,1h), 9.64(s,1h),9.37(s,1h),8.89(s,1h),8.31(s,2h),8.18(dd,j=17.8,11.6hz,1h),7.86(s,1h), 6.36(dd,j=17.8,1.3hz,1h),6.19(dd,j=11.5,1.2hz,1h),5.23(d,j=19.4hz,1h),5.11(d,j =19.4hz,1h),4.55(dd,j=7.4,2.3hz,1h),4.30-4.27(m,1h),3.64(dd,j=7.7,4.0hz,2h), 3.58(s,3h),3.42(s,3h),3.16(s,3h),3.08
–
2.99(m,2h),2.63
–
2.58(m,1h),2.46
–
2.42(m, 2h),2.37
–
2.31(m,1h),2.16(d,j=8.9hz,1h),2.08-2.04(m,1h),1.79(d,j=7.3hz,3h),1.64 (t,j=7.2hz,2h),1.59(t,j=7.6hz,3h),0.17(s,1h),-2.04(s,1h).
13
c nmr(151mhz, dmso-d6)δ:195.26,172.09,171.94,171.81,164.30,161.40,161.06,153.76,149.69,147.81, 144.34,140.57,136.93,135.60,134.99,134.72,131.52,129.90,128.93,127.60,122.57,105.92, 103.75,96.21,93.61,51.29,49.41,47.53,37.79,32.39,32.27,30.16,24.45,22.88,18.41,17.35, 11.93,11.52,10.63.hrms(maldi-tof)m/z:calcd for c
39h44
n9o5s2[m]
782.29013,found 782.28722.
[0051]
[实施例5]
[0052]
n-[5-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-5-氧代戊基]焦脱镁叶绿酰胺(ii4)的制备:
[0053][0054]
采用化合物i的合成方法制备了化合物ii4。1h nmr(600mhz,dmso-d6)δ:9.17(s,1h), 8.90(s,1h),8.23(d,j=10.2hz,2h),7.68(dd,j=16.8,10.0hz,1h),7.55(t,j=6.7hz,1h), 6.50(d,j=0.9hz,1h),5.96(dd,j=10.0,2.1hz,1h),5.55(dd,j=16.8,2.1hz,1h),4.38(pd,j =6.8,1.0hz,1h),4.31(q,j=6.9hz,1h),3.60(dq,j=16.2,8.0hz,1h),3.41(d,j=15.7hz, 1h),3.32(dq,j=16.1,8.0hz,1h),3.07(q,j=6.9hz,2h),2.48
–
2.37(m,4h),2.32(s,2h), 2.27(s,2h),2.12(s,2h),2.01(q,j=7.1hz,2h),1.66
–
1.50(m,4h),1.38(t,j=8.0hz,3h), 1.08(d,j=6.7hz,3h).
13
c nmr(151mhz,dmso-d6)δ:197.41,173.72,172.07,170.88,169.58, 168.66,154.28,153.88,153.12,151.01,144.85,141.23,136.53,136.46,136.22,136.04,135.92, 131.17,129.58,129.06,121.72,106.03,100.08,99.03,94.02,50.26,49.40,43.18,38.47,37.65, 34.35,28.82,28.14,24.53,20.21,19.47,17.42,12.22,11.92,11.55.hrms(maldi-tof)m/z: calcd for c
39h44
n8o4s2[m]
795.2985,found 795.2960。
[0055]
[实施例6]
[0056]
n-[6-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-6-氧代己基]焦脱镁叶绿酰胺(ii5)的制备:
[0057][0058]
采用化合物i的合成方法制备了化合物ii5。1h nmr(600mhz,dmso-d6)δ:9.17(s,1h), 8.90(s,1h),8.23(d,j=10.3hz,3h),7.68(dd,j=16.8,10.0hz,1h),7.42(t,j=6.7hz,1h), 6.50(d,j=0.9hz,1h),5.96(dd,j=10.0,2.1hz,1h),5.55(dd,j=16.8,2.1hz,1h),4.38(pd,j =6.8,1.1hz,1h),4.31(q,j=6.9hz,1h),3.60(dq,j=16.2,8.0hz,1h),3.41(d,j=15.8hz, 2h),3.38
–
3.26(m,1h),3.07(q,j=7.0hz,2h),2.42(td,j=7.3,1.1hz,4h),2.32(s,2h),2.27 (s,2h),2.12(s,2h),2.01(q,j=7.1hz,2h),1.61(p,
j=7.1hz,2h),1.57-1.48(m,2h),1.43
–ꢀ
1.33(m,5h),1.09(d,j=6.7hz,3h).
13
c nmr(151mhz,dmso-d6)δ:197.41,173.72,172.07, 170.88,169.58,168.66,154.28,153.88,153.12,151.01,144.85,141.23,136.22,135.92,131.17, 129.58,129.06,121.72,106.03,100.08,99.03,94.02,50.26,49.40,43.18,39.35,37.65,34.35, 28.99,28.14,25.59,25.13,20.21,19.47,17.42,12.22,11.92,11.55.hrms(maldi-tof)m/z: calcd for c
40h46
n8o4s2[m]
809.3142,found 809.3117。
[0059]
[实施例7]
[0060]
n-[7-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-7-氧代庚基]焦脱镁叶绿酰胺(ii6)的制备:
[0061][0062]
采用化合物i的合成方法制备了化合物ii6。1h nmr(600mhz,dmso-d6)δ:9.17(s,1h), 8.90(s,1h),8.23(d,j=10.3hz,2h),7.68(dd,j=16.8,10.0hz,1h),7.50(t,j=6.7hz,1h), 6.50(d,j=0.9hz,1h),5.96(dd,j=10.0,2.1hz,1h),5.55(dd,j=16.8,2.1hz,1h),4.43
–
4.28(m,2h),3.60(dq,j=16.2,8.0hz,1h),3.42(d,j=16.3hz,1h),3.32(dq,j=16.1,8.0hz, 1h),3.08(q,j=6.9hz,2h),2.42(td,j=7.3,3.7hz,4h),2.32(s,2h),2.27(s,2h),2.12(s,2h), 2.01(q,j=7.1hz,2h),1.63(p,j=7.1hz,2h),1.53
–
1.44(m,2h),1.41
–
1.28(m,7h),1.09(d, j=6.7hz,3h).
13
c nmr(151mhz,dmso-d6)δ:197.41,173.79,172.05,170.88,169.58,168.66, 154.28,153.88,153.12,150.99,144.85,141.29,136.53,136.46,136.22,136.04,135.92,131.17, 129.51,129.06,121.77,106.03,100.08,99.03,94.02,50.26,49.40,43.18,39.35,37.65,34.35, 29.10,28.62,28.14,27.31,25.55,20.21,19.47,17.42,12.22,11.92,11.55.hrms(maldi-tof) m/z:calcd for c
41h48
n8o4s2[m]
823.3293,found 823.3273。
[0063]
[实施例8]
[0064]
n-[8-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-8-氧代辛基]焦脱镁叶绿酰胺(ii7)的制备:
[0065][0066]
采用化合物i的合成方法制备了化合物ii7。1h nmr(600mhz,dmso-d6)δ:9.17(s,1h), 8.90(s,1h),8.23(d,j=10.3hz,2h),7.68(dd,j=16.8,10.0hz,1h),7.48(t,j=6.7hz,1h), 6.50(d,j=0.9hz,1h),5.96(dd,j=10.0,2.1hz,1h),5.55(dd,j=16.8,2.1hz,1h),4.38(pd,j =6.7,1.0hz,1h),4.32(q,j=6.9hz,1h),3.60(dq,j=16.2,8.0hz,1h),3.42(d,j=16.3hz, 1h),3.38
–
3.26(m,1h),3.08(q,j=7.0hz,2h),2.42(td,j=7.3,3.7hz,4h),2.32(s,2h),2.27 (s,2h),2.12(s,2h),2.01(q,j=7.1hz,2h),1.62(p,j=7.1hz,2h),1.48
–
1.40(m,2h),1.44
–ꢀ
1.37(m,2h),1.38(s,2h),1.36(s,1h),1.35
–
1.22(m,4h),1.09(d,j=6.7hz,3h).
13
c nmr (151mhz,dmso-d6)δ:197.41,173.89,172.05,170.88,169.83,168.66,154.28,153.88,153.12, 150.99,144.85,141.29,136.55,136.46,136.22,136.04,135.92,131.17,129.51,129.06,121.77, 106.03,100.08,98.86,94.02,49.62,49.40,43.18,39.36,37.65,34.35,29.39,29.21,28.62,28.14, 27.31,25.47,20.21,19.47,17.42,12.22,11.92,11.55.hrms(maldi-tof)m/z:calcd forc
42h50
n8o4s2[m]
837.3755,found 837.3730。
[0067]
[实施例9]
[0068]
n-((4-氨磺酰基)苯基)焦脱镁叶绿酰胺(iii1)的制备:
[0069][0070]
采用化合物i的合成方法制备了化合物iii1。1h nmr(600mhz,dmso-d6)δ:9.64(s,1h),7.93 (s,1h),7.72(d,j=17.9hz,5h),7.34(s,1h),6.92(dd,j=16.8,10.0hz,1h),6.68(s,1h),6.00 (d,j=0.9hz,1h),5.68(dd,j=13.7,10.1hz,1h),5.37(dd,j=16.9,13.7hz,1h),4.55(s,2h), 3.89-3.65(m,1h),3.13(dd,j=19.5,1.6hz,1h),3.08
–
2.80(m,4h),2.41(s,3h),2.39
–
2.28(m, 1h),2.26(s,3h),2.10-2.01(m,1h),1.84(s,2h),1.70-1.53(m,1h),1.29(d,j=6.8hz,3h),1.12 (t,j=8.0hz,3h).
13
c nmr(151mhz,dmso-d6)δ:
196.73,172.86,170.78,169.06,155.74, 155.19,151.12,145.02,142.15,140.05,136.32,136.13,135.30,133.99,131.62,130.50, 129.80,128.35,121.41,119.39,103.44,100.74,99.51,93.81,50.69,49.82,47.10,33.90, 28.69,19.44,17.91,17.29,12.07,12.02,11.12.hrms(maldi-tof)m/z:calcd forc
39h40
n6o4s[m]
688.2832,found 688.2865。
[0071]
[实施例10]
[0072]
n-((4-氨磺酰基)苯基甲基)焦脱镁叶绿酰胺(iii2)的制备:
[0073][0074]
采用化合物i的合成方法制备了化合物iii2。1h nmr(600mhz,dmso-d6)δ:9.41(s,1h), 7.85-7.79(m,2h),7.56(s,1h),7.52-7.43(m,3h),7.20(s,1h),6.90-6.80(m,2h),6.02-5.94 (m,2h),5.76(dd,j=13.7,10.1hz,1h),5.38(dd,j=16.8,13.8hz,1h),4.44(s,2h),4.10(dt,j =12.3,1.0hz,1h),3.85(dt,j=12.5,8.4hz,1h),3.72-3.56(m,1h),3.15-3.05(m,1h),2.99(d, j=1.5hz,2h),2.74-2.63(m,1h),2.44(s,3h),2.40
–
2.28(m,1h),2.25(s,3h),2.10-2.03(m, 1h),1.94
–
1.81(m,2h),1.83(s,2h),1.46
–
1.37(m,1h),1.27(d,j=6.8hz,3h),1.15(t,j=8.0 hz,3h).
13
c nmr(151mhz,dmso-d6)δ:196.73,173.51,170.78,169.06,155.74,155.19, 151.12,145.02,143.06,142.15,139.68,136.32,136.13,133.99,131.62,130.50,129.80, 127.74,126.30,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,43.62,34.27, 28.69,19.44,17.91,17.29,12.07,12.02,11.12.hrms(maldi-tof)m/z:calcd forc
40h42
n6o4s[m]
702.2988,found 702.3022。
[0075]
[实施例11]
[0076]
n-(2-((4-氨磺酰基)苯基)乙基)焦脱镁叶绿酰胺(iii3)的制备:
[0077][0078]
采用化合物i的合成方法制备了化合物iii3。1h nmr(600mhz,dmso-d6)δ:9.37(s,1h), 7.83
–
7.74(m,3h),7.38
–
7.32(m,2h),7.06(s,1h),6.86(dd,j=16.8,10.1hz,1h),6.72(s, 1h),5.92(d,j=1.1hz,1h),5.83
–
5.73(m,2h),5.37(dd,j=16.9,13.7hz,1h),4.56-4.32(m, 1h),4.38(s,2h),3.37(td,j=12.3,3.9hz,1h),3.24-3.15(m,1h),3.08(dd,j=19.6,1.1hz,1h), 3.04
–
2.84(m,4h),2.57(td,j=12.3,2.7hz,1h),2.45(s,3h),2.40
–
2.28(m,1h),2.28(s,3h), 1.81(d,j=2.2hz,1h),1.81(s,2h),1.76
–
1.64(m,2h),1.29(d,j=6.8hz,3h),1.10(t,j=8.0 hz,3h).
13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74,155.19, 151.12,147.20,145.02,142.80,142.15,136.32,136.13,133.99,131.62,130.50,129.80, 129.63,126.03,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,41.12,35.24, 34.27,28.69,19.44,17.91,17.29,12.07,12.02,11.12.hrms(maldi-tof)m/z:calcd forc
41h44
n6o4s[m]
716.3145,found 716.3178。
[0079]
[实施例12]
[0080]
n-(3-((4-氨磺酰基)苯基)丙基)焦脱镁叶绿酰胺(iii4)的制备:
[0081][0082]
采用化合物i的合成方法制备了化合物iii4。1h nmr(600mhz,dmso-d6)δ:9.23(s,1h), 7.89
–
7.83(m,2h),7.65(s,1h),7.47
–
7.41(m,2h),7.02(s,1h),6.89(dd,j=16.8,10.0hz, 1h),6.67(s,1h),6.15(d,j=1.1hz,1h),5.76(s,1h),5.39(dd,j=16.8,13.8hz,1h),4.48(s, 2h),3.74-3.62(m,1h),3.63-3.50(m,1h),3.24
–
3.09(m,3h),3.00
–
2.85(m,2h),2.78(pd,j= 6.8,1.0hz,1h),2.73
–
2.65(m,1h),2.43(s,3h),2.39-2.35(m,1h),2.31(s,3h),2.21-2.15(m, 1h),2.08
–
1.97(m,1h),1.92-1.90(m,1h),1.85
–
1.75(m,5h),1.26
(d,j=6.8hz,3h),1.10(t, j=8.0hz,3h).
13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74, 155.19,151.12,145.02,143.61,142.15,136.13,133.99,131.62,130.50,129.80,128.39, 126.25,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,39.84,34.27,33.31, 30.67,28.69,19.44,17.91,17.29,12.07,12.02,11.12.hrms(maldi-tof)m/z:calcd forc
42h46
n6o4s[m]
730.3301,found 730.3335。
[0083]
[实施例13]
[0084]
n-(4-((4-氨磺酰基)苯基)丁基)焦脱镁叶绿酰胺(iii5)的制备:
[0085][0086]
采用化合物i的合成方法制备了化合物iii5。1h nmr(600mhz,dmso-d6)δ:9.17(s,1h), 7.95-7.89(m,2h),7.75(s,1h),7.52
–
7.47(m,2h),7.12(s,1h),6.90(dd,j=16.8,10.0hz,1h), 6.72(s,1h),5.80(dd,j=13.8,10.0hz,1h),5.55
–
5.45(m,3h),4.41(s,2h),4.00(td,j=12.3, 1.8hz,1h),3.55
–
3.44(m,2h),3.31(dt,j=12.4,2.9hz,1h),3.23
–
3.06(m,3h),3.00(dd,j= 19.6,1.3hz,1h),2.71
–
2.59(m,1h),2.46(s,3h),2.41
–
2.18(m,3h),2.04(s,3h),1.95
–
1.85 (m,1h),1.82(d,j=1.2hz,3h),1.80
–
1.55(m,3h),1.18(d,j=6.9hz,3h),1.11(t,j=8.0hz, 3h).
13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74,155.19, 151.12,145.02,143.61,142.15,136.32,136.13,133.99,131.62,130.50,129.80,128.39, 126.25,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,39.55,35.73,34.27, 28.74,28.69,28.06,19.44,17.91,17.29,12.07,12.02,11.12.hrms(maldi-tof)m/z: calcd for c
43h48
n6o4s[m]
744.3458,found 744.3491。
[0087]
[实施例14]
[0088]
n-(5-((4-氨磺酰基)苯基)戊基)焦脱镁叶绿酰胺(iii6)的制备:
[0089]
[0090]
采用化合物i的合成方法制备了化合物iii6。1h nmr(600mhz,dmso-d6)δ:9.34(s,1h), 7.88
–
7.78(m,3h),7.46(dt,j=7.5,1.2hz,2h),7.01(s,1h),6.87(dd,j=16.7,10.1hz,1h), 6.72(s,1h),5.85(d,j=0.9hz,1h),5.82
–
5.70(m,2h),5.37(dd,j=16.9,13.7hz,1h),4.38(s, 2h),4.13-4.02(m,1h),3.90-3.73(m,1h),3.19
–
3.08(m,3h),3.04
–
2.90(m,2h),2.81(pd,j= 6.7,6.2,3.3hz,1h),2.62
–
2.46(m,2h),2.45(s,3h),2.39
–
2.28(m,1h),2.27(s,3h),2.06
–ꢀ
1.94(m,2h),1.81(s,2h),1.92
–
1.56(m,6h),1.27(d,j=6.8hz,3h),1.11(t,j=8.0hz,3h). 13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74,155.19,151.12, 145.02,143.61,142.15,136.13,133.99,131.62,130.50,129.80,128.39,126.25,121.41, 103.44,100.74,99.51,93.81,50.69,49.82,47.10,39.94,35.53,34.27,30.26,29.84,28.69, 26.59,19.44,17.91,17.29,12.07,12.02,11.12.hrms(maldi-tof)m/z:calcd forc
44h50
n6o4s[m]
758.3614,found 758.3648。
[0091]
[实施例15]
[0092]
n-(6-((4-氨磺酰基)苯基)己基)焦脱镁叶绿酰胺(iii7)的制备:
[0093][0094]
采用化合物i的合成方法制备了化合物iii7。1h nmr(600mhz,dmso-d6)δ:9.33(s,1h), 7.85(dd,j=5.5,2.0hz,3h),7.49
–
7.44(m,2h),6.89(s,1h),6.76(s,1h),5.89
–
5.81(m,2h), 5.71(dd,j=13.7,10.1hz,1h),5.35(dd,j=16.7,13.8hz,1h),4.36(s,2h),4.21
–
4.12(m,1h), 4.05(dd,j=19.5,1.1hz,1h),3.67(td,j=12.3,2.9hz,1h),3.12
–
2.97(m,3h),2.89-2.80(m, 1h),2.70
–
2.56(m,3h),2.46(s,3h),2.39
–
2.28(m,1h),2.25(s,3h),2.02
–
1.90(m,2h),1.85
ꢀ–
1.75(m,5h),1.71
–
1.53(m,2h),1.55
–
1.31(m,4h),1.19(d,j=6.8hz,3h),1.12(t,j=8.0 hz,3h).
13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74,155.19, 151.12,145.02,143.61,142.15,136.32,136.13,133.99,131.62,130.50,129.80,128.39, 126.25,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,41.60,35.53,34.27, 33.54,31.12,29.06,28.71,28.69,19.44,17.91,17.29,12.07,12.02,11.12.hrms (maldi-tof)m/z:calcd for c
45h52
n6o4s[m]
772.3771,found 772.3804。
[0095]
[实施例16]
[0096]
n-(7-((4-氨磺酰基)苯基)庚基)焦脱镁叶绿酰胺(iii8)的制备:
[0097][0098]
采用化合物i的合成方法制备了化合物iii8。1h nmr(600mhz,dmso-d6)δ:9.34(s,1h), 7.88
–
7.82(m,2h),7.80(s,1h),7.47(dt,j=7.4,1.1hz,2h),6.97
–
6.86(m,2h),6.73(s,1h), 5.89(d,j=1.1hz,1h),5.76(s,1h),5.69(dd,j=13.7,10.1hz,1h),5.39(dd,j=16.8,13.8hz, 1h),4.36(s,2h),3.92
–
3.81(m,2h),3.18(dd,j=19.6,1.1hz,1h),3.07
–
2.93(m,3h),2.92
–
2.78(m,2h),2.67
–
2.52(m,2h),2.45(s,3h),2.26(s,3h),2.14
–
2.04(m,1h),1.82(s,3h),1.86
ꢀ–
1.73(m,2h),1.73
–
1.49(m,6h),1.46
–
1.31(m,2h),1.28(d,j=6.9hz,3h),1.15
–
0.99(m, 4h).
13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74,155.19, 151.12,145.02,143.61,142.15,136.32,136.13,133.99,131.62,130.50,129.80,128.39, 126.25,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,41.60,35.53,34.27, 33.54,31.52,29.44,28.85,28.69,26.91,19.44,17.91,17.29,12.07,12.02,11.12.hrms (maldi-tof)m/z:calcd for c
46h54
n6o4s[m]
786.3927,found 786.3961。
[0099]
[实施例17]
[0100]
n-(8-((4-氨磺酰基)苯基)辛基)焦脱镁叶绿酰胺(iii9)的制备:
[0101][0102]
采用化合物i的合成方法制备了化合物iii9。1h nmr(600mhz,dmso-d6)δ:9.33(s,1h), 7.87
–
7.77(m,3h),7.46
–
7.40(m,2h),7.35(s,2h),6.94
–
6.81(m,3h),5.84(d,j=0.9hz,1h), 5.77
–
5.66(m,2h),5.35(dd,j=16.8,13.7hz,1h),4.62-4.49(m,1h),3.32-3.12(m,1h),3.09
–ꢀ
3.00(m,1h),2.81
–
2.67(m,4h),2.67
–
2.56(m,1h),2.49(s,3h),2.49-2.44(m,1h),2.41
–
2.29 (m,1h),2.24(s,3h),1.92
–
1.79(m,4h),1.82
–
1.45(m,8h),1.31
–
1.21(m,4h),1.12(t,j=8.0 hz,3h).
13
c nmr(151mhz,dmso-d6)δ:196.73,174.04,170.78,169.06,155.74,155.19, 151.12,145.02,143.61,142.15,136.32,136.13,133.99,
131.62,130.50,129.80,128.39, 126.25,121.41,103.44,100.74,99.51,93.81,50.69,49.82,47.10,41.60,35.53,34.27, 33.54,31.52,29.79,29.14,28.69,26.91,19.44,17.91,17.29,12.07,12.02,11.12.hrms (maldi-tof)m/z:calcd for c
46h54
n6o4s[m]
800.4084,found 800.4117。
[0103]
[实施例18]
[0104]
光敏剂对人乳腺癌mda-mb-231细胞的光动力抗增殖实验
[0105]
受试细胞:人乳腺癌mda-mb-231细胞。
[0106]
光源:xd-635ab型激光器;sd2490型激光功率测量仪。
[0107]
受试化合物:
[0108]
n-(5-氨磺酰基-1,3,4-噻二唑-2-基)焦脱镁叶绿酰胺(i);
[0109]
n-[2-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-2-氧代乙基]焦脱镁叶绿酰胺(ii1);
[0110]
n-[3-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-3-氧代丙基]焦脱镁叶绿酰胺(ii2);
[0111]
n-[4-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-4-氧代丁基]焦脱镁叶绿酰胺(ii3);
[0112]
n-[5-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-5-氧代戊基]焦脱镁叶绿酰胺(ii4);
[0113]
n-[6-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-6-氧代己基]焦脱镁叶绿酰胺(ii5);
[0114]
n-[7-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-7-氧代庚基]焦脱镁叶绿酰胺(ii6);
[0115]
n-[8-((5-氨磺酰基-1,3,4-噻二唑-2-基)氨基)-8-氧代辛基]焦脱镁叶绿酰胺(ii7);
[0116]
n-((4-氨磺酰基)苯基)焦脱镁叶绿酰胺(iii1);
[0117]
n-((4-氨磺酰基)苯基甲基)焦脱镁叶绿酰胺(iii2);
[0118]
n-(2-((4-氨磺酰基)苯基)乙基)焦脱镁叶绿酰胺(iii3);
[0119]
n-(3-((4-氨磺酰基)苯基)丙基)焦脱镁叶绿酰胺(iii4);
[0120]
n-(4-((4-氨磺酰基)苯基)丁基)焦脱镁叶绿酰胺(iii5);
[0121]
n-(5-((4-氨磺酰基)苯基)戊基)焦脱镁叶绿酰胺(iii6);
[0122]
n-(6-((4-氨磺酰基)苯基)己基)焦脱镁叶绿酰胺(iii7);
[0123]
n-(7-((4-氨磺酰基)苯基)庚基)焦脱镁叶绿酰胺(iii8);
[0124]
n-(8-((4-氨磺酰基)苯基)辛基)焦脱镁叶绿酰胺(iii9);
[0125]
acetazolamide-conjugate bodipyphotosensitizer(az-bps);
[0126]
焦脱镁叶绿酸-a;
[0127]
阳性对照药物talaporfin(他拉泊芬)。
[0128]
光动力抗肿瘤细胞增殖作用实验:
[0129]
将处于对数生长期的细胞用胰酶消化后,完全培养基重悬成细胞悬液,随之将其接种于 96孔板,每孔100μl,置于37℃5%co2培养箱培养,24h后加入光敏剂(浓度分别为0,
0.04, 0.12,0.37,1.11,3.33μm);3h后换成新鲜培养基,然后进行光照(功率18mw/cm2,波长 650nm,光剂量4j/cm2);72h时进行mtt检测。培养终止前4h加入20μl 5mg/ml的 mtt,吸弃培养液后加150μl dmso终止反应,酶标仪570nm检测od值。实验重复三次。实验结果见表1,结果表明焦脱镁叶绿酰胺衍生物i和ii1–
ii7以及iii1–
iii9对人乳腺癌细胞均具有显著的抗增殖作用,比阳性对照药物他拉泊芬抑制能力强,与无靶向配体的焦脱镁叶绿酸a相比,其具有靶向性的化合物ii1抑制细胞活性效果好。
[0130]
表1新化合物对人乳腺癌mda-mb-231细胞抗增殖作用
[0131][0132][0133]
*p《0.05与阳性对照药talaporfin相比
[0134]
**p《0.001与阳性对照药talaporfin相比。
再多了解一些
本文用于企业家、创业者技术爱好者查询,结果仅供参考。